Proteomic projects
Protein Biomarkers for Precision Medicine
With the great opportunities that Precision Medicine offers comes the need of novel biomarkers for clinical application. We work towards early diagnosis and improved patient stratification in ovarian- and breast cancer.
The best biomarkers known to date are tumor-derived proteins. To truly realize clinical application of protein novel biomarkers, we do biomarker discovery, verification and validation through developing and using fit for purpose technologies in each step. We combine mass spectrometry-based proteomics and recombinant antibody technology, two powerful and yet complementary technologies for biomarker discovery, verification, validation as well as clinical transition.
AFFIRM
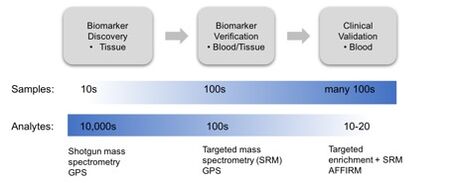
Biomarker discovery is typically performed in human tissue samples. Clinical assays are however preferably performed in an easily accessible fluid such as blood samples. Clinical protein quantification in blood is today measured mainly through enzyme linked immunosorbent assays (ELISA). These are limited because of availability of the well characterized antibody pairs required. Targeted selected reaction monitoring (SRM) MS has high multiplexing capability and has been demonstrated to also provide the reproducibility and precision of proteins in plasma required for clinical use (1 2). SRM analysis alone can provide highly sensitive measurements in the low ng/ml range but this requires extensive sample workup and separation, procedures that are hardly amendable to high-throughput. Targeted enrichment has therefore been combined with SRM readout.
We have over the past years developed the novel and unique AFFIRM analysis platform for targeted protein analysis in complex samples (3). AFFIRM (AFFInity sRM) has capacity for multiplexed targeted protein enrichment by single chain variable fragments (scFv), coupled to magnetic beads with subsequent on-bead trypsin digestion and liquid chromatography (LC)-SRM readout in a semi-automated workflow. In this way, the sensitivity of an antibody is combined with the specificity of the SRM readout to provide assays with multiplexed measurement of target proteins with high sensitivity, reproducibility and throughput. AFFIRM offers:
· Exact protein quantification with absolute specificity and high sensitivity
· Protein isoform/mutant specific readout
· Elucidation of differential post-translational modifications
· Simple handling and automated to save labor time and for precision
· Fast development of assays for novel target proteins and high throughput
· Low cost and direct clinical applicability of the assay format
AFFIRM has capacity to be an assay format directly applicable in a clinical setting; antibody enrichment is routinely used in clinical laboratories through the wide use of ELISA assays, and the exact mass spectrometry instrumentation and instrument settings required for SRM analysis, is already available at clinical laboratories, today employed for measuring small molecules in blood for drug screening. There is thus minimal technology transfer involved to realize such analyses in a real-world setting.
Design, implementation and multisite evaluation of a system suitability protocol for the quantitative assessment of instrument performance in liquid chromatography-multiple reaction monitoring-MS (LC-MRM-MS) Abbatiello, S. E. et al. Mol Cell Proteomics 12, 2623–2639 (2013).
- Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Addona, T. A. et al. Nat Biotechnol 27, 633–641 (2009).
- AFFIRM--a multiplexed immunoaffinity platform that combines recombinant antibody fragments and LC-SRM analysis Säll A, Carlsson F, Olsson N, Wingren C, Ohlin M, Persson H, Waldemarson S. .J Proteome Res 13, 5837–5847 (2014).
