Cytomegalovirus-specific repertoires
Introduction
Cytomegalovirus, in short CMV, is a usually non-pathogenic virus commonly found in the human population. Following infection, the virus persists in the host but the carrier is protected from disease by its immune system. Both cellular and humoral antibody activities are believed to be involved in the defense against such CMV-induced active disease.
The Department of Immunotechnology has had a longstanding interest in research on antibody responses in humans against cytomegalovirus. The research has mainly focused on such antibody responses against the diagnostically important antigen pp65 (lower matrix protein; ppUL83) and the therapeutically interesting surface glycoprotein B (gB; gp58/116; gpUL55).
Biological activity of human antibodies against the major neutralization determinants on CMV gB
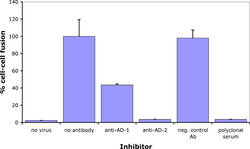
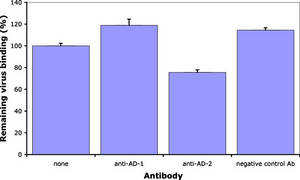
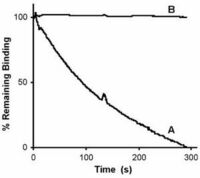
Antibodies against CMV gB has long been known to neutralize virus infection. In collaboration with Prof. Radak we have demonstrated that neutralizing antibodies recognizing AD-1 and AD-2 mediate this effect through different mechanisms. Briefly, an antibody against AD-2 prevents membrane fusion down-stream of virus adsorption to cells.
However, even at concentrations that completely neutralize virus infection and membrane fusion, ITC88 (a human AD-2-specific monoclonal antibody), just as AD-1 specific human antibodies, does not prevent virus adsorption. However, in contrast to anti-AD-1, the AD-2-specific antibody substantially reduced heparin-resistant cellular receptor binding of CMV. Thus, although both antibody types act down-stream of heparan sulfate proteoglycans binding, they differ in their direct mechanism (Gicklhorn et al., 2003).

Diversity
It has been shown that the human antibody response to the major neutralization determinant AD-1 residing in gp58 is highly diverse. Several overlapping epitopes can be found within the AD-1 structure and each epiope (defined as reactivity against specific mutants and protein fragments) may bind both neutralizing and non-neutralizing antibodies (Schoppel et al., 1996; Speckner et al., 1999).
The neutralization determinant AD-2 expressed by the gp116 fragment of gB is also diverse and different antibodies against this epitope have different ultra-fine specificity for the epitope (Lantto et al., 2002a; Ohlin et al., 1996; Silvestri et al., 1993). Overall, very little information on how different antibodies neutralize this virus has been available, largely due to the scarcity of different antibodies against this epitope.
Virus neutralization
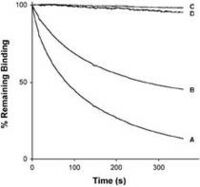
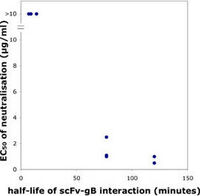
We have, as outlined above, recently shown that the repertoire of antibody genes that is capable of actually creating a AD-2 specific paratope is restricted, a factor which may contribute to the relatively low immunogenicity of this potent virus-neutralization epitope (Lantto et al., 2002a). We have now also demonstrated that a divalent antibody format was required for virus neutralization despite a very slow dissociation of monovalent antibody fragments (scFv) from gB. Thus, size and/or cross-linking is a major factor determining neutralization efficacy of antibodies directed towards the AD-2 epitope (Lantto et al., 2002b).
Furthermore, dissociation rate of the anti-AD2/AD2 complex seems to correlate with efficacy of neutralization. Importantly, it was also shown that certain clinical isolates carrying modifications within the AD-2 epitope may escape neutralization by some members of an AD-2 specific immune repertoire despite the fact that they still bind these antibody fragments (Lantto et al., 2003) suggesting that the interplay between the humoral immune response and the virus is extremely complex.

References
- Barrios et al. (2007) Mol. Immunol. 44, 680-690. Abstract at publisher's website
- Gicklhorn et al. (2003) J. Gen. Virol. 84, 1859-1862. Abstract at publisher's website
- Lantto et al. (2003) Virology 305, 201-209. Abstract at publisher's website
- Lantto et al. (2002a) Eur. J. Immunol. 32, 1659-1669. Abstract at publisher's website
- Lantto et al. (2002b) J. Gen Virol. 83, 2001-2005. Abstract at publisher's website
- Speckner A et al. (1999) J. Gen. Virol. 80, 2183-2191. Abstract at publisher's website
- Ohlin et al. (1996) Mol. Immunol. 33, 47-56 Abstract at publisher's website
- Schoppel et al. (1996) Virology 216, 133-145 Abstract at publisher's website
- Silvestri et al. (1993) Serodiagn. Immunother. Infect. Disease 5, 209-216
- Ohlin et al. (1993) J. Virol. 67, 703-710. Abstract at publisher's website
Repertoires of antibodies against the neutralization epitope AD-2 expressed by CMV glycoprotein B. Definition of critical features of a gB specific antibody binding site
When shuffling the light chain of the AD-2 specific antibody ITC88 with a light chain library derived from peripheral blood B cells, it was apparent that this antibody only could use a very restricted group of light chain sequences closely related to the sequence used by the original hybridoma.
aIt is thus suggested that in vivo developed specific antibody VH sequences may display very limited light chain promiscuity. Furthermore, when introducing these light chain sequences the ultra-fine specificity against the AD-2 epitope was sometimes slightly modified without introducing multireactive properties to this antibody. The chain shuffling approach may thus develop new ultrafine specificities not previously found among available monoclonal antibodies but which at least in this case had been observed in polyclonal antibody preparations. These results define the basis for the light chain restriction in this antibody.
In an extension to these studies, the criteria for forming an AD-2 specific paratope in an antibody was further defined. Importantly we have been able to demonstrate the features of a high affinity repertoire against the potently neutralising determinant AD-2 on gB and described possible reasons for the poor immunogenicity of this structure. Mutations of residues in CDRH1 and CDRL1 were critical determinants in allowing an AD-2 specific antibody to gain a high affinity character in its recognition of intact CMV gB.
The sequences of variable region sequences from the early studies are available in GenBank under accession codes: L37300-L37309. Additional sequences are available in GenBank under accession codes: F443427-AF443433 and in GenBank under accession code: AF492001.
References:
- Ohlin et al. (1996) Mol. Immunol. 33, 47-56.
- Lantto et al. (2002) Eur. J. Immunol. 32, 1659-1669.
- Ohlin (2014) Mol Immunol. 60, 95-102.
Engineered complement fixation in CMV gB-specific antibodis
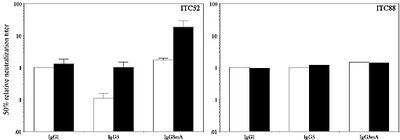
We have developed a range of recombinant antibodies against glycoprotein B epitopes AD-1 and AD-2 with different levels of complement activating potential. These antibodies, which carried IgG1, IgG3 and IgG3mA (a synthetic version of IgG3 carrying a shorter and less flexible hinge), were evaluated for their potential to use complement to neutralise virus infection. The following results were obtained:
- Antibodies recognising the AD-2 epitope of CMV gB were not affected by which constant domains they used and their neutralising potential was not improved by the presence of human complement.
Antibodies recognising the AD-1 epitope showed a marked effect of the constant region on the neutralising capacity. The more flexible IgG3 version of ITC52 was less potent in neutralising the virus as compared to IgG1 and IgG3mA in the absence of complement. The activity of IgG1 was not affected by the presence of complement. In contrast, both IgG3 and IgG3mA enhanced their virus-neutralising activity in the presence of complement, making the IgG3mA-version of ITC52 approximately 8-fold more potent than its IgG1 counterpart in neutralising the virus. Thus, selecting an appropriate isotype of an AD-1-specific antibody for use in therapy may enhance its anti-viral properties and thus the efficacy of the treatment.
References
- Furebring et al. (1997) Gene 188, 191-198.
- Furebring C et al. (2001) Mol. Immunol. 38, 833-840.
Antibody variable region usage of repertoires specific for the pp65 antigen as well as the AD-1 epitope on glycoprotein B.
Antibodies against the major CMV components pp65 and gB are highly diverse in their sequences and they use a wide range of germline sequences. It has, however, been observed that there appears to be an overrepresentation of VH5 segment usage among antibodies specific for the AD-1 epitope of gB.
All these antibodies seem to be mutated in comparison to germline sequences suggesting that these immune responses are T cell dependent. Although these antibodies in some cases uses germline genes sometimes associated with auto- or multi reactive properties, no such activity has been demonstrated. Furthermore, these CMV-specific antibodies do not express substantial levels of idiotypes frequently associated with these germline genes and such auto-/multi reactive antibodies.
The sequences of these variable region sequences are available in GenBank under accession codes: L26531-L26540, L26890-L26896, L26898 and L37310-L37311
References
- Rioux et al. (1995) Immunol. Infect. Dis. 5, 43-52.
- Ohlin et al. (1994) Mol. Immunol. 31, 983-991.
Reagents for collaboration
During the coarse of these investigations, we have developed a number of specific, high affinity, human monoclonal antibodies recognizing cytomegalovirus pp65 and gB. In addition one human antibody recognizing pp28 has been obtained. These reagents are available for non-commercial purposes to researchers on a collaborative basis upon request to Mats Ohlin.
Since the 1990-ies our research has utilized recombinant DNA technology to investigate and develop human antibodies specific for CMV. Consequently, a large panel of variants of some of the antibodies described below are available. Furthermore, to faciitate the development of neutralizing antibodies specific for CMV we have established and validated a semi-high throughput system for screening specific binders developed through combinatorial antibody library technology (Carlsson et al., 2012).
Specificity CMV gB AD-1
Clones: ITC33, ITC39, ITC48, ITC52, ITC63B
References:
- Ohlin et al. (1993) J. Virol. 67, 703-710. (Abstract)
- Ohlin et al. (1994) Mol. Immunol. 31, 983-991. (Abstract)
- Schoppel et al. (1996) Virology 216, 133-145 (Abstract)
- Speckner et al. (1999) J. Gen. Virol. 80, 2183-2191. (Abstract)
- Furebring et al (2002) Mol. Immunol. 38, 833-840. (Abstract)
- Gicklhorn et al. (2003) J. Gen. Virol. 84, 1859-1862. (Abstract)
- Barrios et al. (2007) Mol. Immunol. 44, 680-690. (Abstract)
- Axelsson et al. (2009) N Biotechnol 25, 429-436. (Abstract)
Specificity CMV gB AD-2
Clones: ITC88 and variants thereof
References:
- Ohlin et al. (1993) J. Virol. 67, 703-710. (Abstract)
- Silvestri et al. (1993) Serodiagn. Immunother. Infect. Disease 5, 209-216.
- Ohlin et al. (1996) Mol. Immunol. 33, 47-56 (Abstract)
- Furebring et al (2002) Mol. Immunol. 38, 833-840. (Abstract)
- Lantto et al. (2002) Eur. J. Immunol. 32, 1659-1669. (Abstract)
- Lantto et al. (2002) J. Gen Virol. 83, 2001-2005. (Abstract)
- Lantto et al. (2003) Virology 305, 201-209. (Abstract)
- Gicklhorn et al. (2003) J. Gen. Virol. 84, 1859-1862. (Abstract)
- Axelsson et al. (2007) Vaccine 26, 41-46. (Abstract)
- Axelsson et al. (2009) N Biotechnol 25, 429-436. (Abstract)
- Carlsson et al. (2012) J Immunol Methods 376, 69-78. (Abstract)
- Ohlin (2014) Mol Immunol 60, 95-102. (Abstract)
Specificity CMV pp65
Clones: MO53, MO58, MO61, MO81
References:
- Ohlin et al. (1991) Clin. Exp. Immunol. 84, 508-514. (Abstract)
- Rioux et al. (1995) Immunol. Infect. Dis. 5, 43-52.
- Ohlin et al. (1995) Clin. Diagn. Lab. Immunol. 2, 325-329.(Abstract)
Specificity CMV pp28
Clones: MO80
References:
- Ohlin et al. (1991) Clin. Exp. Immunol. 84, 508-514. (Abstract)
Specificity unknown
Clones: MO69
References:
- Ohlin et al. (1991) Clin. Exp. Immunol. 84, 508-514. (Abstract)

